Buffer Solution Bio Def
A buffer solution more precisely pH buffer or hydrogen ion buffer is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or vice versa. An example of a.
 The Blood Buffer System Youtube
The Blood Buffer System Youtube
What is the Structure of Nephron and its Functions.

Buffer solution bio def. Acidic buffer solutions are those that have strong acids and weak bases as their components. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. A solvent is a substance that dissolves another substance by pulling the molecules apart through electrochemical interactions.
They help in a neutralization reaction to a certain extent. A buffer is a solution or a substance that has the ability to maintain pH and bring it back to its optimal value. Buffer Solution Definition Biology A buffer solution more precisely pH buffer or hydrogen ion buffer is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or vice versaIts pH changes very little when a small amount of strong acid or base is added to it.
An example of a common buffer is a solution of acetic acid CH 3 COOH and sodium acetate. An acidic buffer solution is simply one which has a pH less than 7. If you add an acid or a base to a buffered solution its pH will not change significantly.
Buffer solutions are solutions in water that mark the combination of acids and bases. Buffer capacity is the amount of acid or base that can be added before the pH of a buffer changes. An example of a buffer solution is bicarbonate in blood which maintains the bodys internal pH.
Types of Buffer Solutions Buffers are broadly divided into two types acidic and alkaline buffer solutions. 1 chemistry A buffer solution. Buffers working in the body fluid adjust the pH level of the blood and function to lower pH if its level rises above 74 by making the blood slightly more acidic 1 3.
A solution containing either a weak acid and a conjugate base or a weak base and a conjugate acid used to stabilize the pH of a liquid upon dilution. A buffer solution more precisely pH buffer or hydrogen ion buffer is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or vice versa. Most buffers consist of a weak acid and a weak base.
In this way a biological buffer helps maintain the body at the correct pH so that biochemical processes continue to run optimally. It does this by the additional or removal of hydrogen ions. Buffer solutions are used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications.
A buffering agent is a weak acid or weak base that helps maintain the pH of an aqueous solution after adding another acid or base. Note- A lot of biological chemical reactions need a constant pH for the reaction to proceed. It is the main buffer in blood plasma and consists of bicarbonate HCO 3 and carbonic acid H 2 CO 3.
Its pH changes very little when a small amount of strong acid or base is added to it and is thus used to prevent a solution s pH change. A solution is a homogeneous mixture of solvent and solute molecules. Its pH changes very little when a small amount of strong acid or base is added to it.
A buffer consists of a weak acid and its conjugate base or a weak base and its conjugate acid. Buffer in chemistry solution usually containing an acid and a base or a salt that tends to maintain a constant hydrogen ion concentration. Ions are atoms or molecules that have lost or gained one or more electrons.
It is used to prevent any change in the pH of a solution regardless of solute. A buffer solution is one which resists changes in pH when small quantities of an acid or an alkali are added to it. Acidic buffer solutions are commonly made from a weak acid and one of its salts - often a sodium salt.
A buffer is an aqueous solution that has a highly stable pH. A buffer consists of a weak acid and its conjugate base or a weak base and its conjugate acid. For instance one of the buffers that maintain the pH of human blood involves carbonic acid H.
A buffers pH changes very little when a small amount of strong acid or base is added to it. Define Buffer Solution Biology A buffer is an aqueous solution used to keep the pH of a solution nearly constant. Buffers typically consist of an acid-base pair with the acid and base differing by the presence or absence of a proton a conjugate acid-base pair.
DEFINITION A buffer is an aqueous solution consisting of a mixture of a weak acid its salt or a weak base its salt that resist a change in pH on the addition of either acid or base. For example a mixture of acetic acid and sodium acetate acts as a buffer solution with a pH of about 475. The bicarbonate buffer neutralizes stronger dietary and metabolic acids HA converting them into weak bases A with the increase in H 2 CO 3.
Buffers A buffer is an aqueous solution containing a weak acid and its conjugate base or a weak base and its conjugate acid. Buffer capacity is the amount of acid or base that can be added before the pH of a buffer changes. Its pH changes very little when a small amount of strong acid or base is added to it.
Acidic buffers are solutions that have a pH below 7 and contain a weak acid and one of its salts. A buffer is an aqueous solution that consists of a mixture of a weak acid and its salt acid buffer or a weak base with its salt basic buffer. A buffer is an aqueous solution used to keep the pH of a solution nearly constant.
The solute then diffuses through the solvent until the concentration is equal in all parts of the solution.
 Buffer Solution Preparation Of Buffer Solution Acidic Basic Buffer Buffer Action Buffer Solution Solutions Electron Configuration
Buffer Solution Preparation Of Buffer Solution Acidic Basic Buffer Buffer Action Buffer Solution Solutions Electron Configuration
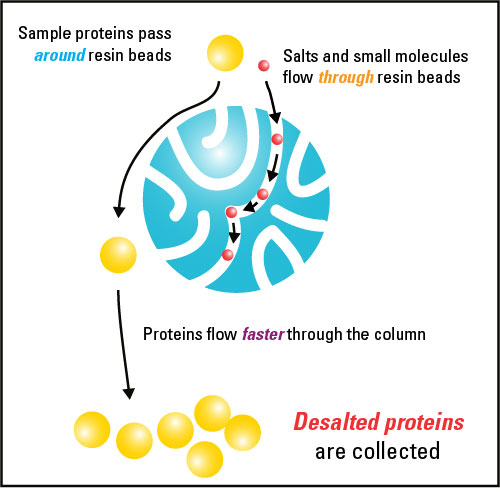 Desalting And Buffer Exchange Wikipedia
Desalting And Buffer Exchange Wikipedia
 What Is A Biological Buffer And How To Choose The Best Buffer For Your Experiment Goldbio
What Is A Biological Buffer And How To Choose The Best Buffer For Your Experiment Goldbio
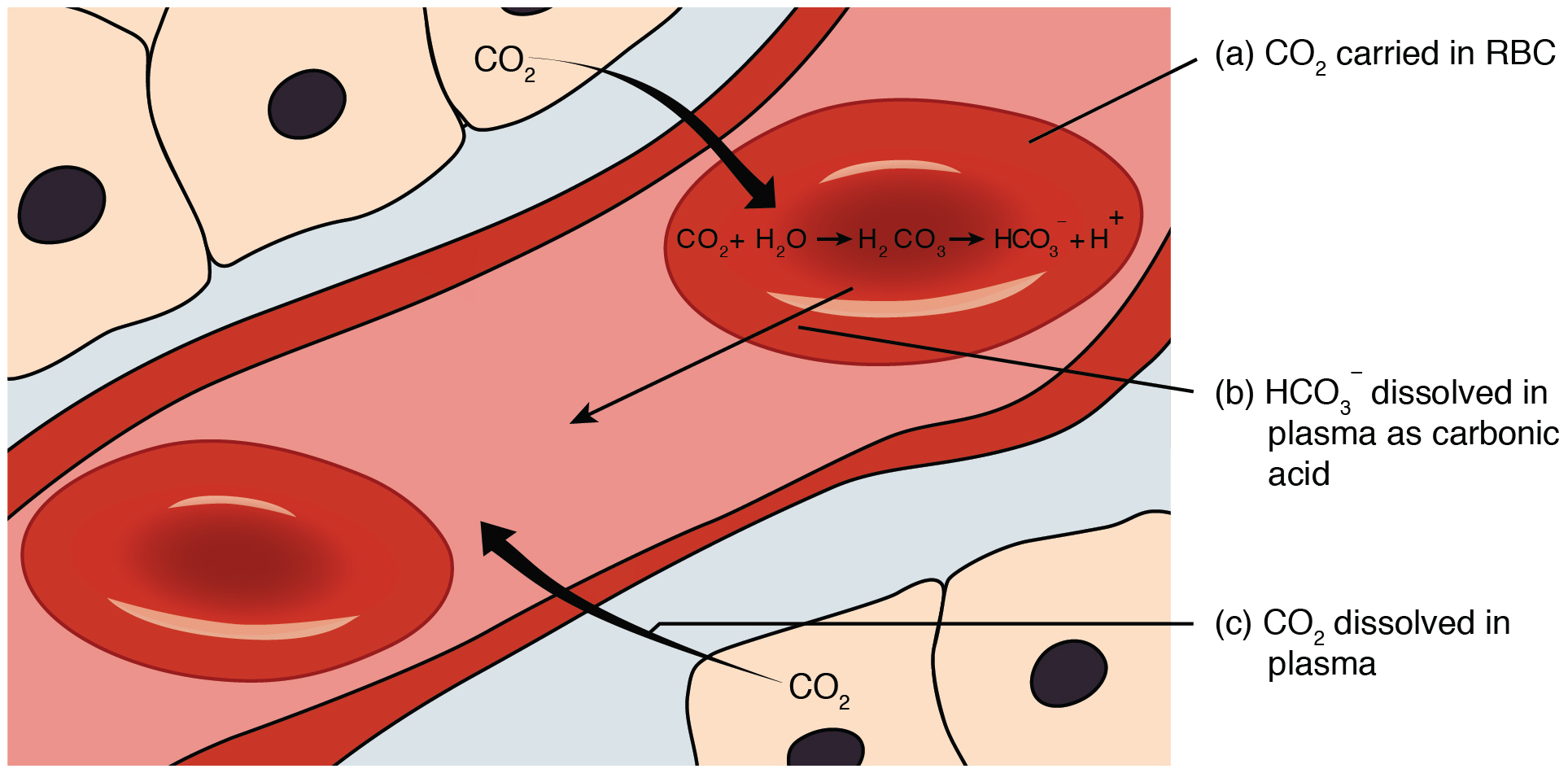 Bicarbonate Buffer System Wikipedia
Bicarbonate Buffer System Wikipedia
 Buffer Solutions Definition Types Preparation Examples And Videos
Buffer Solutions Definition Types Preparation Examples And Videos
 Buffer Stock Definition Economics Online Economics Online
Buffer Stock Definition Economics Online Economics Online
 How To Build Resilience Experiments In Wellness Emotional Resilience How To Build Resilience Coping Skills
How To Build Resilience Experiments In Wellness Emotional Resilience How To Build Resilience Coping Skills
 What Is Buffer Solution Full Explain In Urdu Hindi Chemistry 11 Learning 4u Youtube
What Is Buffer Solution Full Explain In Urdu Hindi Chemistry 11 Learning 4u Youtube
 Buffer Solutions Biochemistry The Biology Notes
Buffer Solutions Biochemistry The Biology Notes
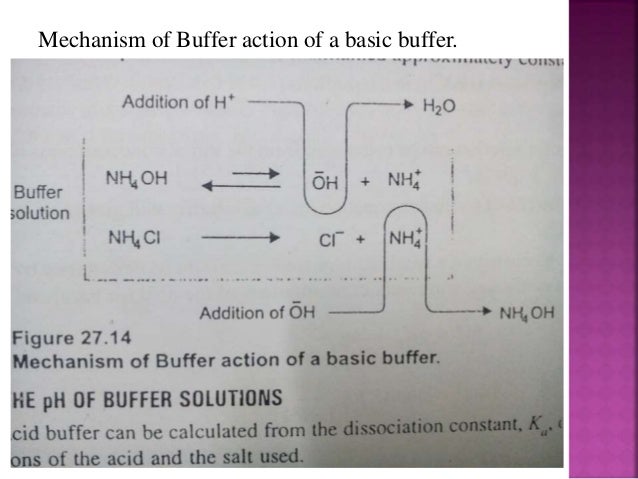
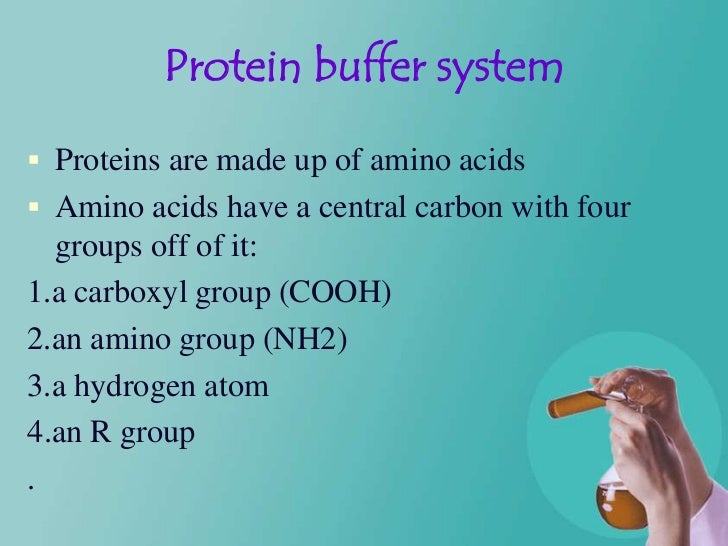
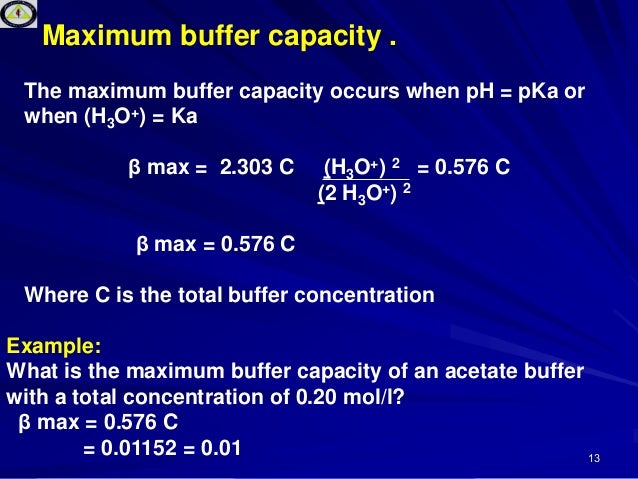
 Lec9 Level4 Debiologicalbuffer 130202064553 Phpapp01
Lec9 Level4 Debiologicalbuffer 130202064553 Phpapp01
1 3 Introduction Water And Buffers Biology Libretexts
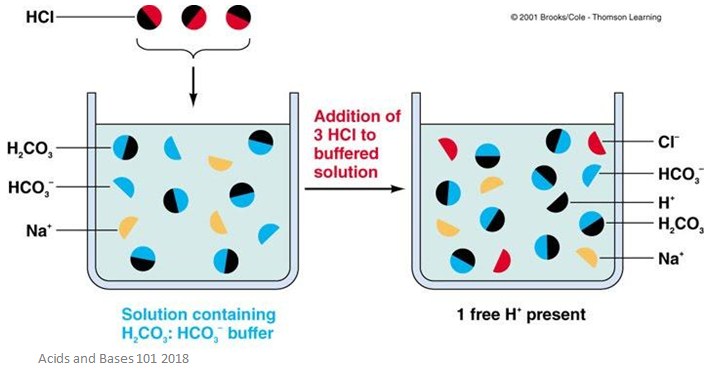 Acid Base Buffers Facts Summary Definition Chemistry Revision
Acid Base Buffers Facts Summary Definition Chemistry Revision
 Ph And Pka Relationship For Buffers Video Khan Academy
Ph And Pka Relationship For Buffers Video Khan Academy